how to find overall order of reaction
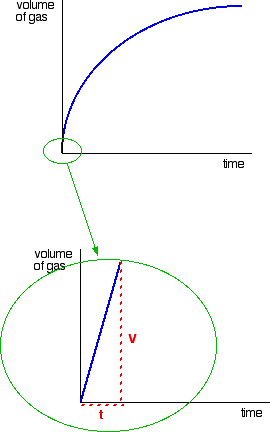
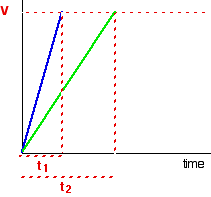
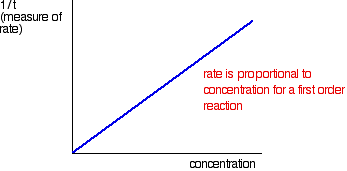
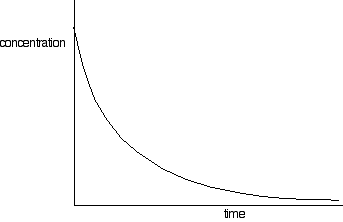
| FINDING ORDERS OF REACTION EXPERIMENTALLY This folio is an introduction to some of the experimental methods that can be used in school labs to observe orders of reaction. There are ii fundamentally unlike approaches to this - y'all can either investigate what happens to the initial rate of the reaction as you change concentrations, or y'all tin can follow a detail reaction all the mode through, and process the results from that unmarried reaction. We will look at these two approaches separately. Don't await total practical details. This is going to be a very long page. I wouldn't actually recommend that you endeavour to read it all in 1 become. Initial rate experiments How initial rate experiments work An outline of the experiments The simplest initial rate experiments involve measuring the time taken for some easily recognisable outcome to happen very early in a reaction. This could include the time taken for, say, 5 cm3 of gas to be produced. Or it could exist the fourth dimension taken for a pocket-size measurable amount of precipitate to exist formed. Or you could measure the time taken for some dramatic colour change to occur. We volition look at examples of all these beneath. You and so change the concentration of ane of the components of the reaction, keeping everything else abiding - the concentrations of other reactants, the total volume of the solution and the temperature and then on. And then you notice the fourth dimension taken for the same event to take place with that new concentration. This is repeated for a range of concentrations of the substance you are interested in. You would demand to cover a reasonably wide range of concentrations, taking perhaps 5 or and then dissimilar concentrations varying from the original 1 down to half of it or less. Obviously, you could then repeat the process by changing something else - the concentration of a different substance, or the temperature, for example. Understanding the results We volition accept a uncomplicated example of an initial charge per unit experiment where you have a gas being produced. This could be a reaction between a metallic and an acrid, for example, or the catalytic decomposition of hydrogen peroxide. If you plotted the volume of gas given off confronting fourth dimension, y'all would probably get the get-go graph below. A measure out of the rate of the reaction at any betoken is establish by measuring the slope of the graph. The steeper the gradient, the faster the rate. Since we are interested in the initial charge per unit, we would demand the slope at the very starting time. If you and so look at the second graph, enlarging the very beginning of the get-go bend, y'all will meet that it is approximately a straight line at that bespeak. That is but a reasonable approximation if you are considering a very early on stage in the reaction. The further into the reaction yous go, the more the graph volition start to curve. Measuring the slope of a straight line is very piece of cake. The slope in this case is simply V/t. Now suppose you did the experiment once again with a unlike (lower) concentration of the reagent. Again, we will measure the time taken for the same volume of gas to be given off, and then nosotros are still only looking at the very beginning of the reaction: The initial rates (in terms of volume of gas produced per second) are: At present suppose you didn't really know what the volume V was. Suppose, for example, that instead of measuring the time taken to collect five cm3 of gas, you just nerveless the gas up to a marker which y'all had fabricated on the side of a test tube. Does it matter? If y'all are simply wanting to compare initial rates, then it doesn't thing. If you look at the expressions in the tabular array above, you should recognise that the initial charge per unit is inversely proportional to the time taken. In symbols: In experiments of this sort, you often only employ 1/t as a mensurate of the initial rate without any farther calculations. You can and then plot 1/t as a measure of rate confronting the varying concentrations of the reactant you are investigating. If the reaction is first order with respect to that substance, then you would get a direct line. That's considering in a first order reaction, the charge per unit is proportional to the concentration. If y'all go a curve, then it isn't commencement order. It might exist second order - just it could equally well have some sort of fractional order similar ane.5 or 1.78. The all-time way effectually this is to plot what is known as a "log graph". The maths of this might non be familiar to y'all, just you may discover that you are asked to do this every bit a function of a applied exam or applied do. If it is an exam, you would probably be given help as to how to go about it. The maths goes similar this: If you take a reaction involving A, with an guild of n with respect to A, the rate equation says:
If you take the log of each side of the equation, yous get:
If you plotted log(charge per unit) agains log[A], this second equation would plot equally a directly line with gradient due north. If you measure the slope of this line, you get the order of the reaction. So you would convert all the values you had for charge per unit into log(rate). Catechumen all the values for [A] into log[A], and then plot the graph. This should exist a straight line. If it isn't, then y'all accept done something incorrect! Measure the slope to find the order, n. | |
| Note:Don't worry if you lot don't understand logs (logarithms), or how I got from the kickoff equation to the 2d one! I suspect that in the unlikely event of you lot needing it in an exam at this level, it would be given to you lot. All you lot need to do is discover the log button on your computer and utilise it to convert your numbers. Exercise to offset with by trying to discover log 2. Information technology should requite a value of 0.3010(etc). Y'all probably have to enter 2 and then press the log push, just on some calculators it might be the other way around. If you do it the wrong way around, you lot will just get an fault message. | |
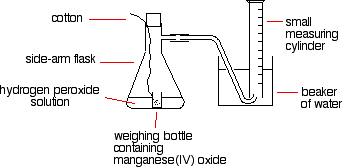
| Some sample reactions The catalytic decomposition of hydrogen peroxide This is a simple example of measuring the initial rate of a reaction producing a gas. A unproblematic ready-up to exercise this might be: The reason for the weighing bottle containing the catalyst is to prevent introducing errors at the beginning of the experiment. Since this is the part of the reaction you lot are most interested in, introducing errors here would be stupid! Yous have to notice a style of adding the catalyst to the hydrogen peroxide solution without changing the volume of gas collected. If you lot added it to the flask using a spatula, and and so speedily put the hurl in, you lot might lose some gas before you got the bung in. Alternatively, as you pushed the bung in, y'all might forcefulness some air into the measuring cylinder. Either way, it makes your results meaningless. To get-go the reaction, yous simply need to shake the flask so that the weighing canteen falls over, and then continue shaking to make sure the catalyst mixes evenly with the solution. You could likewise use a special flask with a divided bottom, with the goad in one side, and the hydrogen peroxide solution in the other. They are easy to mix by tipping the flask. If you use a ten cm3 measuring cylinder, initially full of water, yous can reasonably accurately tape the time taken to collect a small fixed book of gas. You lot could, of course, use a small gas syringe instead. If you were looking at the event of the concentration of hydrogen peroxide on the rate, then y'all would take to change its concentration, but keep everything else constant. The temperature would take to be kept constant, so would the total volume of the solution and the mass of manganese(Iv) oxide. You would also have to be certain that the manganese(IV) oxide used always came from the aforementioned canteen then that its state of partition was always the same. You could, of course, utilize much the aforementioned apparatus to find out what happened if y'all varied the temperature, or the mass of the catalyst, or the state of division of the catalyst. The thiosulphate-acid reaction If you lot add together dilute muriatic acid to sodium thiosulphate solution, you get the slow formation of a pale yellow precipitate of sulphur. In that location is a very simple, but very constructive, way of measuring the time taken for a small fixed amount of precipitate to class. Stand the flask on a slice of paper with a cantankerous drawn on it, and then expect downwardly through the solution until the cross disappears. So . . . y'all put a known volume of sodium thiosulphate solution in a flask. Then you add a modest known volume of dilute hydrochloric acid, start timing, swirl the flask to mix everything upward, and stand up information technology on the paper with the cross on. Fourth dimension how long it takes for the cross to disappear. So repeat using a smaller volume of sodium thiosulphate, but topped up to the same original volume with water. Everything else should be exactly as before. If you started with, say, 50 cmthree of sodium thiosulphate solution, yous would repeat the experiment with perhaps, 40, 30, 20, 15 and 10 cm3 - each time fabricated up to a total of 50 cm3 with water. The actual concentration of the sodium thiosulphate doesn't take to be known. In each example, you could record its relative concentration. The solution with xl cm3 of sodium thiosulphate solution plus 10 cm3 of water has a concentration which is eighty% of the original 1, for example. The 1 with 10 cmiii of sodium thiosulphate solution plus forty cm3 of water has a concentration which is 20% of the original one. When you lot came to plotting a rate against concentration graph, every bit we looked at farther up the page, you would plot ane/t every bit a mensurate of the rate, and book of sodium thiosulphate solution as a mensurate of concentration. Alternatively, you lot could plot relative concentrations - from, say, 20% to 100%. Information technology doesn't actually matter - the shape of the graph will be identical. Yous could too expect at the effect of temperature on this reaction, by warming the sodium thiosulphate solution earlier you added the acid. Take the temperature afterwards adding the acrid, though, because the cold acid will absurd the solution slightly. This fourth dimension yous would change the temperature between experiments, but keep everything else constant. To become reasonable times, you lot would take to use a diluted version of your sodium thiosulphate solution. Using the full strength solution hot will produce enough precipitate to hibernate the cantankerous almost instantly. Iodine clock reactions There are several reactions which go under the name "iodine clock". They are all reactions which give iodine as one of the products. This is the simplest of them, only only because it involves the most familiar reagents. The reaction we are looking at is the oxidation of iodide ions by hydrogen peroxide under acidic conditions. The iodine is formed first as a pale yellow solution darkening to orange and and then dark crimson, before nighttime grey solid iodine is precipitated. There is a very clever mode of picking out a when a detail very minor corporeality of iodine has been formed. Iodine reacts with starch solution to give a very deep blue solution. If you added some starch solution to the reaction above, every bit before long as the first trace of iodine was formed, the solution would turn blue. That doesn't actually help! Nevertheless, iodine also reacts with sodium thiosulphate solution. If yous add together a very pocket-sized amount of sodium thiosulphate solution to your reaction mixture (including the starch solution), it will react with the iodine that is initially produced, and and so the iodine won't affect the starch, and yous won't go whatsoever blue colour. However, when that modest amount of sodium thiosulphate has been used upwards, there is nix to stop the next lot of iodine produced from reacting with the starch. The mixture suddenly goes blue. | |
| Note:In that location is a neat piece of video on YouTube showing an iodine clock reaction (not necessarily the one I am talking about here, simply it doesn't affair). It shows iii reactions side by side:
The blueish colours appear in exactly the order you would predict. | |
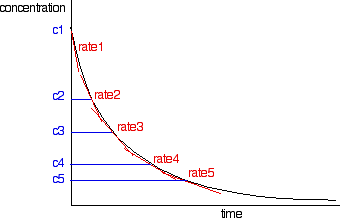
| In our instance, you lot could evidently look at the effect of changing the hydrogen peroxide concentration, or the iodide ion concentration, or the hydrogen ion concentration - each fourth dimension, of class, keeping everything else constant. That would let y'all find the orders with respect to everything taking function in the reaction. Post-obit the course of a single reaction Rather than doing a whole ready of initial charge per unit experiments, you tin likewise get information about orders of reaction by following a particular reaction from start to finish. At that place are two different ways yous tin can exercise this. You lot can take samples of the mixture at intervals and do titrations to discover out how the concentration of one of the reagents is changing. Or (and this is much easier!) you tin can measure some physical property of the reaction which changes every bit the reaction continues - for case, the volume of gas produced. We need to look at these two different approaches separately. Sampling the reaction mixture Bromoethane reacts with sodium hydroxide solution as follows: During the course of the reaction, both bromoethane and sodium hydroxide will go used upward. Notwithstanding, it is relatively easy to measure the concentration of the sodium hydroxide at any one time by doing a titration with some standard acid - for example, with hydrochloric acid of a known concentration. You start with known concentrations of sodium hydroxide and bromoethane, and usually it makes sense to take them both the aforementioned. Because the reaction is 1:1, if the concentrations outset the aforementioned as each other, they will stay the same as each other all through the reaction. And so all you need to do is to accept samples using a pipette at regular intervals during the reaction, and titrate them with standard muriatic acid in the presence of a suitable indicator. That is a lot easier said than done! The trouble is that the reaction will still be going on in the time it takes for you to practise the titration. And, of course, you but go one endeavor at the titration. By the fourth dimension you take another sample, the concentration of everything will accept changed! There are ii ways effectually this. You tin can slow the reaction downwards by diluting it, adding your sample to a larger volume of cold water before you do the titration. And so do the titration as quickly equally possible. That's virtually effective if you lot are doing your reaction at a temperature above room temperature. Cooling it likewise as diluting it will slow it down even more. But if possible (and information technology is possible in the example nosotros are talking about) it is amend to cease the reaction completely before you practise the titration. In this case, you tin stop it by adding the sample to a known book (called to exist an backlog) of standard muriatic acid. That will use up all the sodium hydroxide in the mixture so that the reaction stops. Now y'all would titrate the resulting solution with standard sodium hydroxide solution, so that you lot tin can find out how much hydrochloric acid is left over in the mixture. That lets y'all calculate how much was used up, and and so how much sodium hydroxide must take been present in the original reaction mixture. This sort of technique is known as a back titration . These calculations tin be quite confusing to practice without some guidance. If y'all are interested, y'all volition find back titrations discussed on pages 72-75 of my chemistry calculations book. Processing the results Yous volition end upwardly with a set of values for concentration of (in this case) sodium hydroxide against time. The concentrations of the bromoethane are, of class, the same as these if you lot started with the same concentrations of each reagent. You can plot these values to give a concentration-fourth dimension graph which volition look something like this: Now it all gets pretty ho-hum! Yous need to find the rates of reaction at a number of points on the graph, and you do this past cartoon tangents to the graph, and measuring their slopes. You would and then draw upwards a simple table of charge per unit against concentration. The quickest way to get on from here is to plot a log graph as described further upwardly the page. Yous would catechumen all your rates into log(rate), and all the concentrations into log(concentration). And so plot log(charge per unit) confronting log(concentration). The slope of the graph gives you the order of reaction. In our case of the reaction between bromoethane and sodium hydroxide solution, the social club would turn out to be 2. Notice that this is the overall order of the reaction - not just the order with respect to the reagent whose concentration you were measuring. The rate of reaction was falling because the concentrations of both of the reactants were falling. | |
| Note:This all takes ages to do - not just the applied which would probably take at least an hr, but all the graph cartoon, and processing the results from the graphs. In that location is no obvious mode this could be asked in any normal applied or written exam at this level. I tin meet that information technology is simply possible that y'all might be asked in principle how you would do it, but actually doing it could only reasonably be a part of a coursework exercise. | |
| Following the grade of the reaction using a physical property An example where a gas is given off A familiar example of this is the catalytic decomposition of hydrogen peroxide that we have already looked at above as an example of an initial rate experiment. This time, yous would measure the oxygen given off using a gas syringe, recording the volume of oxygen collected at regular intervals. So the practical side of this experiment is straightforward, but the adding isn't. The problem is that you are measuring the volume of production, whereas to find an order of reaction you have to be working in terms of the concentration of the reactants - in this case, hydrogen peroxide. That means that you will take to work out the concentration of hydrogen peroxide remaining in the solution for each volume of oxygen you record. To practise this, yous have to be happy with calculations involving the ideal gas law, and likewise basic mole calculations. Having got a table of concentrations against time, you volition and then procedure them in exactly the same mode every bit I described in a higher place. Plot the graph, describe tangents to notice rates at various concentrations, and then plot a log graph to find the gild. | |
| Annotation:Information technology seems to me fairly unlikely that you could always be asked to do this in an test state of affairs. And, at this level, you would nigh certainly be given some guidance with the calculations In a applied exam, few schools could provide a class set of the very expensive gas syringes authentic enough to produce meaningful results, and the fourth dimension taken to procedure the results would be far greater than was available in any normal examination. That is every bit true of a theory paper. If y'all know that you lot tin can follow the class of a reaction which produces a gas using this method, that is probably all you will need. Only check your syllabus, and past papers and marker schemes. | |
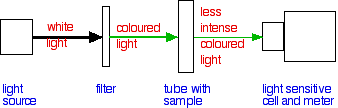
| Colorimetry In whatsoever reaction involving a coloured substance (either reacting or beingness produced), you can follow the course of the reaction using a colorimeter. All of this is independent in one fairly small box. The colour of the calorie-free can be changed by selecting a particular coloured filter (or using some more than sophisticated device like a diffraction grating). The colour is chosen then that it is the frequency of light which is absorbed by the sample. Taking copper(II) sulphate solution as a familiar example, you lot would cull to use a red filter, because copper(2) sulphate solution absorbs red lite. The more full-bodied the solution is, the more of the red light information technology will blot. | |
| Note:For an explanation of why absorbing carmine light makes copper(Two) sulphate solution blue, see the start part of the folio about the colours of complex metallic ions. You don't need to read about the origin of the colour for now. | |
| A commonly quoted example of the use of colorimetry in rates of reaction is the reaction between propanone and iodine in the presence of an acid catalyst. The solution of iodine in propanone starts off brown, and then fades through orange to xanthous to colourless every bit the iodine is used up. A colorimeter lets you measure out the corporeality of light which is absorbed every bit it passes through a solution - recorded equally the absorbance of the solution. It is mutual to plot a calibration bend for a colorimeter past making up solutions of the coloured substance of known concentration and then measuring the absorbance of each nether the aforementioned conditions as you lot will do the experiment. You and then plot a graph of absorbance against concentration to give your scale curve. During your rate of reaction experiment, yous read the absorbance from the meter at regular intervals, and so use your calibration curve to convert those values into concentrations. And so you are faced with the same graphical methods as earlier. | |
| Note:In truth, these days, you are more likely to plug your colorimeter into a estimator with the right software to do information technology all for you! | |
| Ii other methods pH measurements If you lot have a reaction in which hydrogen ions are reacting or being produced, in principle you should be able to follow changes in their concentration using a pH meter. You lot may exist enlightened that pH is a measure out of hydrogen ion concentration, and it isn't difficult to calculate an actual hydrogen ion concentration from a pH. However, if you are measuring pH over a fairly narrow range of hydrogen ion concentrations, the pH doesn't change all that much. For instance, the pH of a solution containing 0.2 mol dm-iii H+ has a pH of 0.70. By the time that the concentration has fallen to 0.one mol dm-three, the pH has only increased to 1.00. Whether it is feasible to use a pH meter obviously depends on how accurate information technology is. If the pH meter only recorded to 0.1 pH units, your results aren't going to exist very good. Conductivity measurements The electric conductivity of a liquid depends on the number of ions present, and the nature of the ions. For instance, we looked at this reaction much further upwards the page: During the form of the reaction, as hydrogen ions and iodide ions go used up, the electrical conductivity of the mixture will autumn. | |
| Note:I am non giving whatsoever more detail on this, because conductivity measurements aren't a part of whatever of the syllabuses that I am tracking. CIE expect you to know that it is possible to utilize conductivity measurements to follow the course of a reaction involving changes in the ions present, only not how you would actually conduct out the experiments or process the results. | |
To the rates of reaction menu . . . To the Physical Chemistry menu . . . To Main Menu . . . © Jim Clark 2011 (modified Oct 2013) | |
Source: https://chemguide.co.uk/physical/basicrates/experimental.html
Posted by: williamsuniagard.blogspot.com

0 Response to "how to find overall order of reaction"
Post a Comment